WHAT WE SEEK TO UNDERSTAND
How the physical shape of an organ emerges from the collective activity of its constituents - thousands of microscopic fluctuating biological cells? To address this question, we develop quantitative human stem-cell systems that undergo complex shape changes in response to minimal biochemical cues.
PROJECTS
Mechanics of Neural tube Formation

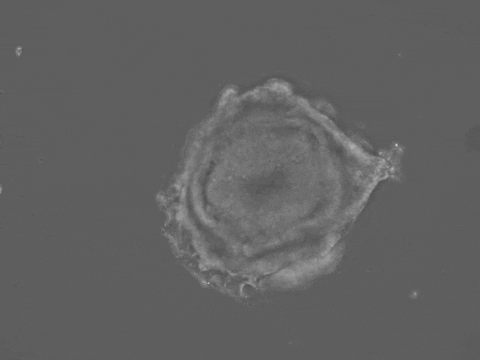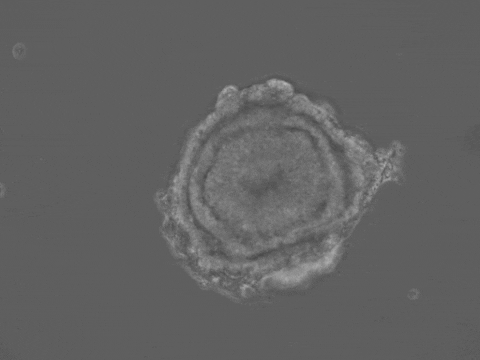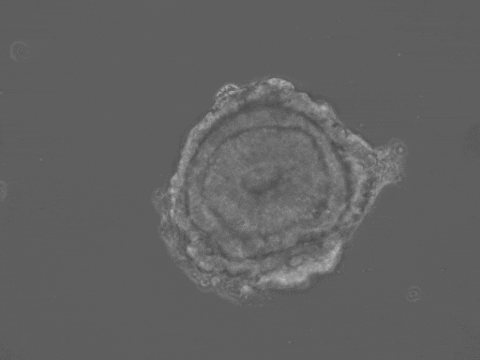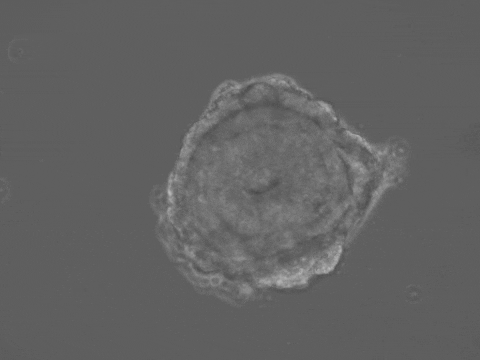
Brain and spinal cord development begin with the folding of the embryonic neural tissue into a tube. Many of the molecular and cellular processes which drive neural tube folding have been elucidated in animal models. However, understanding how these microscopic activities integrate to drive forces and shape changes at the tissue scale remains a challenge. To address this issue, we developed a human stem-cell system that self-organizes into a neural tube in a dish. Our system offers quantitative control over experimental parameters, and a unique opportunity to understand the biomechanics of human neural tube formation in health and disease.
Human Birth Defects
Failures in neural tube folding are among the most common birth defects, affecting 1:1000 pregnancies. Neural tube defects result in severe disabilities and lethality shortly after birth. Surprisingly, genetic mutations associated with neural tube defects in humans do not lead to defects in mice. Our stem-cell platform offers a unique opportunity to study how neural tube morphogenesis is regulated by the human genome and to understand its evolution across species.

Synthetic Morphogenesis

Organ shape information is encoded in genetic networks and in the boundary and initial conditions of the tissue. Our 3D stem-cell sheets are a clean slate on which we can quantitatively explore this idea. We will study how tissue geometry, mechanical environment, and positioning of morphogens control tissue morphogenesis. We aim to reveal 'morphogenetic motifs' which will allow us to design new synthetic organ forms.




.png)